French pharmaceuticals distribution platform Apodis Pharma leaking 1.7+ TB of confidential data
Security Affairs
DECEMBER 1, 2020
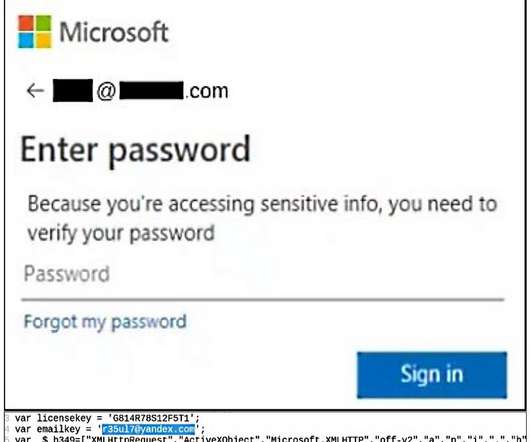
The CyberNews investigation team discovered French pharmaceuticals distribution platform Apodis Pharma leaking 1.7+ Apodis Pharma is a company that offers a digital supply chain management platform and other software solutions created for pharmacies, healthcare institutions, pharmaceutical laboratories, and health insurance companies.





































Let's personalize your content