Why IDMP compliance requires adaptive data governance
Collibra
MAY 5, 2022
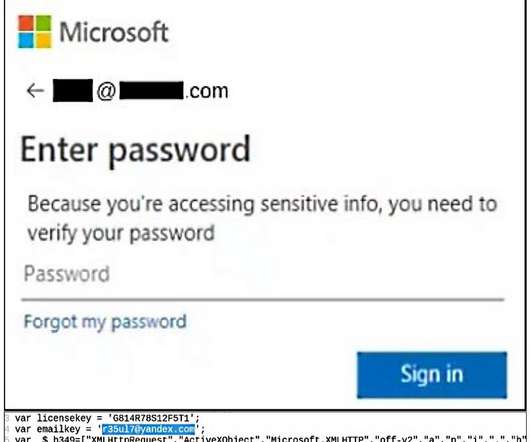
Pharmaceutical, biotech and medical devices firms, among others, must comply with a plethora of regulations. For instance, at the end of October 2021, the European Medicines Agency (EMA) communicated a change in the implementation strategy for IDMP, requiring a more agile and data-centric approach. They can use the right value sets.



























Let's personalize your content